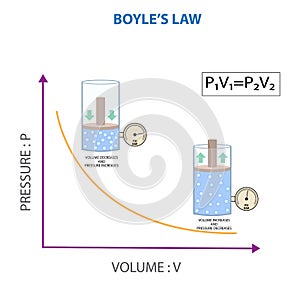
boyle law relationship between pressure and volume gas constant temperature
|
||||||||||||||
|
|
|
|
||||||||||||
|
||||||||||||||
Stockphotos.ro (c) 2025. All stock photos are provided by Dreamstime and are copyrighted by their respective owners.